PREGNANCY
This is the second of a series of two articles regarding musculoskeletal clinical issues and phycological change in the women body during and after pregnancy and how exercises can help to reduces the symptom of these ones.
The aim of this article is to answer questions that some women may have and help them to find solution to some recurrent pregnancy problem through exercises. In any case, if you are pregnant, GO SEE your doctor BEFORE engaging any kind of physical activity. I’m not a doctor and this article only relate the most relevant finding regarding pregnancy and the effect of physical activity during and after the gestational period.
Anatomical and physiological adaptations to pregnancy during each trimester
v Musculoskeletal adaptations
One of the major musculoskeletal adaptation that body go through during pregnancy is the expansion of the uterus. This expansion displaces the center of gravity of the body, which results in the woman compensating to avoid falling forward. This may result in progressive lumbar lordosis and anterior rotation of the pelvis on the femur.1
If there is an increase in lordosis, then an increase in anterior flexion of the cervical spine and abduction of the shoulders also takes place.1
During pregnancy, the anterior tilt of the pelvis increases by approximately 5°, followed with an increase in hip flexion during stance phase, an increase of knee flexion during the terminal stance phase, a decrease of knee extension, and a decrease of ankle dorsiflexion and plantar flexion.1
Muscle Change :
Changes in hormone receptors and their regulators may promote changes in skeletal muscle fibre type (from oxidative (type I) to glycolytic type (Type II or IIx))1
Balance :
Postural balance is affected after the first trimester of pregnancy. Subsequently, falling is a common cause of injury in the general pregnant population, and pregnant women are 2–3 times more likely to be injured by falling than are non-pregnant women. Physical activity and exercise may mitigate this risk, but this has not been tested in the research setting.1
Cardiorespiratory, metabolic and thermoregulatory adaptations to pregnancy
v Cardiorespiratory adaptations to pregnancy
From about the fifth week of gestation, pregnancy induces rapid, progressive and substantial alterations to the cardiovascular system, which ensureblood supply to the fetus.1
Estrogen-mediated remodeling reduces vascular tone, leading to a primary reduction in afterload and an increase in venous capacitance.
This is reflected in increased resting cardiac output of about 50% comparatively to non-pregnant women.1
Moreover, remodelling of the heart increases the dimensions of the ventricular cavity without increasing wall thickness, increases aortic capacitance and reduces peripheral vascular resistance. There is a 15–20 bpm increase in resting HR comparatively to non-pregnant women.1
Stroke volume also increases by approximately 10% by the end of the first trimester. This manifests before significant enhancement in maternal blood volume, which may increase up to 50% above pre-pregnancy volume by late pregnancy.1
Futhermore, there are also pregnancy-induce adaptations to the maternal respiratory system. For example, remodelling and expansion of the thoracic cage raises diaphragmatic mid-position; this decreases residual volume and expiratory reserve volume. 1
One of the most substantial physiological pregnancy-induced changes, possibly designed to protect against fetal acidosis, is an increase in respiratory sensitivity to carbon dioxide (CO2 ) early in pregnancy. This increases tidal volume and minute ventilation, which reduces arterial carbon dioxide tension and increases arterial oxygen tension. These changes create a buffer that protects the fetus from any acute elevations in
maternal carbon dioxide levels.1
On the other hand, many pregnant women complain of respiratory discomfort (dyspnea), especially in late pregnancy, both at rest and after exertion. Resting oxygen uptake (relative–mL/kg/min) reflects the increase in body mass during pregnancy, and thus declines slightly during each trimester. However, during submaximal steady-state exercise, pregnant women’s perceptions of respiratory effort and dyspnea seem to be reduced, because maternal anatomical and mechanical respiratory adaptations reduce airway resistance, preserve breathing mechanics, minimize the effort of ventilation and thus increase minute ventilation.1
v Effect of posture on maternal and fetal cardiovascular dynamics
As mentioned above, while pregnant, the body go through a series of musculoskeletal adaptation which will have an impact also on the physiological level. Thus, supine posture leads to compression of the inferior vena cava by the pregnant uterus, which, in turn, decreases stroke volume, end-diastolic volume index and left ventricular ejection time, with decelerations in maternal HR. Being motionless (including standing and certain yoga positions) or exercising in the supine position, may decrease venous return and cause hypotension in 10–20% of pregnant women.1
Metabolic adaptations in a normal pregnancy
v Thermoregulatory adaptation to pregnancy
During neural tube development, raising body core temperature above 103°F (39°C) can increase the risk of fetal (neural tube defect) abnormalities. But, the fetal neural tube is formed 35–42 days from the last menstrual period, and therefore exposure to increases in temperature after this time should not affect the risk of neural tube defects.
Exercising in pregnancy at 60–70% of VO2 max in a controlled environment for up to 60 min does not raise core temperature above 38°C. Higher body core temperature could be reached during strenuous exercise, such as marathon running, or exercising outdoors in hot and humid weather. 1
Thermoregulation steadily improves during pregnancy, as reflected by a gradual decline in rectal temperature. As pregnancy advances, a downward shift in body temperature threshold initiates sweating, resulting in evaporative heat loss starting at a lower body temperature. The improved heat dissipation at rest may be due to decreased vascular tone, with increased circulation to the skin, augmentation of minute ventilation and plasma volume expansion. Maternal heat dissipation is vital because fetal metabolism generates heat, and fetal temperature depends on maternal temperature, fetal metabolism and uterine blood flow (figure 1).1
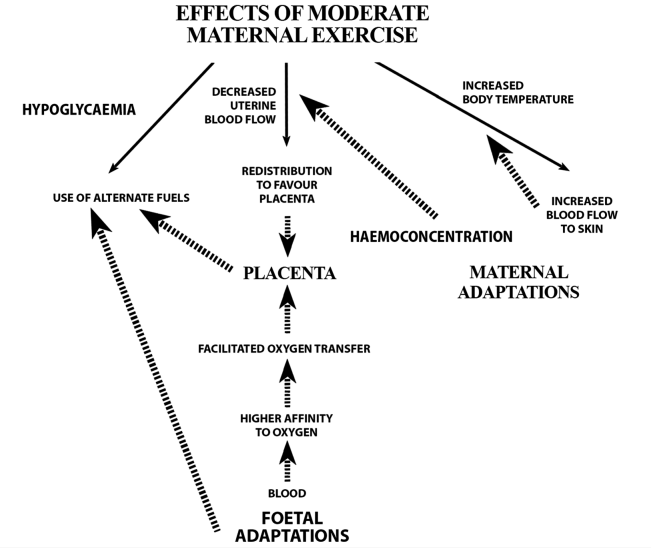
Figure 1: Flow chart of maternal, placental and fetal adaptations that occur in a low-risk pregnancy to protect the fetus from potential risks of maternal exercise. The solid arrows represent potential effects of maternal exercise. The dashed arrows represent fetal, placental and maternal adaptations that occur in a low-risk pregnancy to counterbalance these potential maternal exercise effects (adapted with permission from Mottola).1
v Nutrional requirements for normal pregnancy
Additional energy is needed specifically for development of the fetus, placenta, amniotic fluid, uterus, breasts, adipose tissue, and the increased volumes of blood and extracellular fluid. The additional energy need for pregnant women with a mean Gestational Weight Gain of 12 kg is estimated to be:
▸ 325 MJ
(77 700 kcal) in total and 375 kJ/day (90 kcal/day) for the first trimester,
▸ 1200
kJ/day (287 kcal/day) for the second trimester, and
▸ 1950
kJ/day (466 kcal/day) for the third trimester of pregnancy.
An exercising woman can monitor whether she has appropriate energy intake by comparing her weight gain and body mass index (BMI) with the Institute of Medicine (IOM) recommendations (table 1).
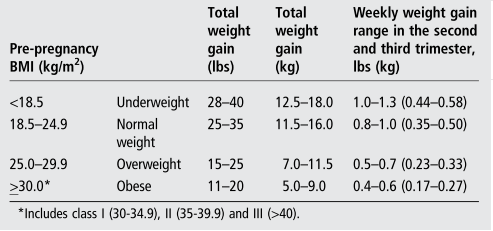
Table 1 : Recommendation for total weight gain range in singleton pregnancies by pre-pregnancy body mass index (BMI) 1
v Endurance, resistance and flexibility training during pregnancy
Endurance :
Overall, studies indicate that a woman’s aerobic fitness will stay the same or improve slightly during pregnancy if she continues to exercise as her maternal symptoms permit. 1
Among recreational athletes, there were no differences in aerobic fitness (absolute VO2 max ) tested during the past 2 months of a singleton pregnancy and again 6–8 weeks post-partum. In more highly conditioned athletes, a moderate-to-high level of exercise during and after pregnancy may lead to an increase in VO 2max in the region of 5–10% after pregnancy. Improved anaerobic working capacity is also better preserved in fitter subjects.1
Borg’s Scale :
A woman who uses Borg’s ratings of perceived exertion (RPE) as a guide may be exercising at a much higher HR than her RPE would suggest. If she is trying to keep her HR within a ‘safe’ range, accordingly to her doctor recommendation, RPE should not be used as the only measure of exercise intensity, particularly from the second trimester. The athlete should measure HR directly.1
During pregnancy, RPE scale does not correlate strongly with HR. HR predicted from RPE is significantly underestimated in the second trimester during walking (ie, actual mean HR is greater than predicted HR by 16 bpm), aerobics classes (15 bpm) and circuit training (18 bpm). In the third trimester, HR while cycling and during aerobics classes are underestimated by a mean of 16 and 11 bpm, respectively, but maximal individual HR underestimations may be up to 54 bpm. 1
Strength Training :
Light-to-moderate
weight training with free weights or weight machines generally has no adverse health effects during pregnancy. Large strength gains have been reported in apparently healthy pregnant women who adopted strength training twice per week for 12 weeks during pregnancy:
— 36% for leg press
— 39% for leg curl
— 39% for lat pull down
— 41% for lumbar extension
–56% for leg extension.
–14% increase in lumbar endurance.1
For women that were following a more intense training regimen before pregnancy an who are considering heavy strength training in pregnancy should understand that the Valsalva manoeuvre used during weight training precipitates a rapid increase in blood pressure and intra-abdominal pressure, and therefore may temporarily decrease blood flow to the fetus. The repercussions to the fetus of these temporary changes remain unknown. 1
In addition, those athletes participating in heavy strength training should acknowledge that large increases in intra-abdominal pressure may harm the pelvic floor support, which may increase the risk of urinary (UI) or anal incontinence (AI) or pelvic organ prolapse (POP) during or after pregnancy. 1
Flexibility Training :
Articulatory injuries may occur during pregnancy due to the Owing to increased levels of relaxin during pregnancy. It has been stated that pregnant women are more lax and have more joint instability.1
v Sport to avoid during pregnancy
High-risk sports can be divided into those with risk of trauma (eg, from a collision or being hit by something (eg, hockey stick) or falling), and those with physiological risk factors (eg, scuba diving).In relation to maternal trauma, placental abruption leading to acute or chronic fetal hypoxia or death, may occur.1
Risks also exist in sports where there may be collision or sudden deceleration. An exhaustive list cannot be created, but examples include bobsledding, luge, equestrian (eg, eventing) activities, pole vaulting, ice hockey and downhill ski. 1
In relation to physiological risk, pregnant women should refrain from diving, because the fetus is not protected from decompression problems and is at risk of malformation and gas embolism after decompression disease.1
Reference
1-Bø K, et al. Br J Sports Med 2016;50:571–589. doi:10.1136/bjsports-2016-096218
Related articles:
Artal R, Wiswell R, Romem Y, et al. Pulmonary responses to exercise in pregnancy. Am J Obstet Gynecol 1986;154:378–83.
Bessinger RC, McMurray RG. Substrate utilization and hormonal responses to exercise in pregnancy. Clin Obstet Gynecol 2003;46:467–78.
Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 2000;71(5 Suppl):1256S–61S.
Butte NF, King JC. Energy requirements during pregnancy and lactation. Public Health Nutr 2005;8:1010–27.
Clapp JF III. The changing thermal response to endurance exercise during pregnancy. Am J Obstet Gynecol 1991;165(Pt 1):1684–9.
Clapp JF III, Capeless E. The VO2max of recreational athletes before and after pregnancy. Med Sci Sports Exerc 1991;23:1128–33.
Cong J, Fan T, Yang X, et al. Structural and functional changes in maternal left ventricle during pregnancy: a three-dimensional speckle-tracking echocardiography study. Cardiovasc Ultrasound 2015;13:6.
Dumas GA, Reid JG. Laxity of knee cruciate ligaments during pregnancy. J Orthop Sports Phys Ther 1997;26:2–6.
Duvekot JJ, Cheriex EC, Pieters FA, et al. Early pregnancy changes in hemodynamics and volume homeostasis are consecutive adjustments triggered by a primary fall in systemic vascular tone. Am J Obstet Gynecol 1993;169: 1382–92.
Gilson GJ, Samaan S, Crawford MH, et al. Changes in hemodynamics, ventricular remodeling, and ventricular contractility during normal pregnancy: a longitudinal study. Obstet Gynecol 1997;89:957–62.
Hartmann S, Bung P. Physical exercise during pregnancy—physiological considerations and recommendations. J Perinat Med 1999;27:204–15.
Heenan AP, Wolfe LA. Plasma acid-base regulation above and below ventilatory threshold in late gestation. J Appl Physiol 2000;88:149–57.
Ibrahim S, Jarefors E, Nel DG, et al. Effect of maternal position and uterine activity on periodic maternal heart rate changes before elective cesarean section at term. Acta Obstet Gynecol Scand 2015;94:1359–66.
Jensen D, Webb KA, Wolfe LA, et al. Effects of human pregnancy and advancing gestation on respiratory discomfort during exercise. Respir Physiol Neurobiol 2007;156:85–93.
Jeffreys RM, Stepanchak W, Lopez B, et al. Uterine blood flow during supine rest and exercise after 28 weeks of gestation. Br J Obstet Gyneacol 2006;113:1239–47.
Knuttgen HG, Emerson K Jr. Physiological response to pregnancy at rest and during exercise. J Appl Physiol 1974;36:549–53.
Lontay B, Bodoor K, Sipos A, et al. Pregnancy and Smoothelin-like Protein 1 (SMTNL1) deletion promote the switching of skeletal muscle to a glycolytic phenotype in human and mice. J Biol Chem 2015;290:17985–98
O’Connor PJ, Poudevigne MS, Cress ME, et al. Safety and efficacy of supervised strength training adopted in pregnancy. J Phys Act Health 2011;8:309–20.
Olson D, Sikka RS, Hayman J, et al. Exercise in pregnancy. Curr Sports Med Rep 2009;8:147–53.
O’Neill ME, Cooper KA, Mills CM, et al. Accuracy of Borg’s ratings of perceived exertion in the prediction of heart rates during pregnancy. Br J Sports Med 1992;26:121–4.
Palatini P, Mos L, Munari L, et al. Blood pressure changes during heavy-resistance exercise. J Hypertens Suppl 1989;7:S72–3.
Pivarnik J. Cardiovascular responses to aerobic exercise during pregnancy and postpartum. Sem Perinatol 1996;20:242–9.
Pivarnik JM, Lee W, Miller JF, et al. Alterations in plasma volume and protein during cycle exercise throughout pregnancy. Med Sci Sports Exerc 1990;22:751–5.
Soultanakis HN, Artal R, Wiswell RA. Prolonged exercise in pregnancy: glucose homeostasis, ventilatory and cardiovascular responses. Semin Perinatol 1996;20:315–27.
Szymanski LM, Satin AJ. Strenuous exercise during pregnancy: is there a limit? Am J Obstet Gynecol 2012;207:179.e1–6.
Vladutiu CJ, Evenson KR, Marshall SW. Physical activity and injuries during pregnancy. J Phys Act Health 2010;7:761–9.
Weissgerber TL, Wolfe LA. Physiological adaptation in early human pregnancy: adaptation to balance maternal-fetal demands. Appl Physiol Nutr Metab 2006;31:1–11.
Wolfe LA, Weissgerber TL. Clinical physiology of exercise in pregnancy: a literature review. J Obstet Gynaecol Can 2003;25:473–83.
Wong SC, McKenzie DC. Cardiorespiratory fitness during pregnancy and its effect on outcome. Int J Sports Med 1987;8:79–83.